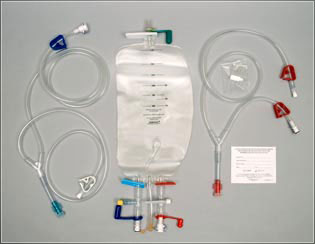
Intraoperative Autologous Blood Salvage and The Hemobag®
Human errors such as misidentifying a patient's blood type and a unit of donor blood present much more of a risk than transmissible diseases.
Intraoperative autologous blood salvaging has been used for many years. Because of on-going safety concerns with the blood supply, a priority remains avoiding transfusion-related adverse events, especially in cardiothoracic and vascular surgery in which blood usage has traditionally been high. Several medical devices have been developed to assist in salvaging the patient's own blood in the perioperative setting. The Hemobag® technology is one.
Background
Providing safe blood for transfusion remains a challenge despite advances in preventing transmission of hepatitis B, hepatitis C, AIDS/HIV, West Nile Virus (WNV), and transfusion-transmitted bacterial infection. Human errors such as misidentifying patients and drawing blood samples from the wrong person present much more of a risk than transmissible diseases.
Additional risks include transfusion-related acute lung injury, a potentially life-threatening condition with symptoms such as dyspnea, fever, and hypotension occurring within hours of transfusion (TRALI), and transfusion-associated immunomodulation (TRIM), which may cause adverse effects such a small increase in the risk of postoperative infection.
Other risks such as variant Creutzfeldt Jakob Disease (vCJD), an invariably fatal disease, remain worrisome. Blood centers worldwide have instituted criteria to reject donors who may have been exposed to vCJD. Screening for transmissible diseases and deferral policies for vCJD designed to improve safety have contributed to shrinking the donor pool. Blood shortages exist in the United States and worldwide. In many industrialized countries 5% or less of the eligible population are blood donors.
As a result, the global medical community has increasingly moved from allogeneic blood (blood collected from another person) towards autologous infusion, in which patients receive their own blood. Another impetus for autologous transfusion is the position of Jehovah's Witnesses on blood transfusion. For religious reasons Jehovah's Witnesses will not accept any allogeneic transfusions from a volunteer's blood donation, but may accept the use of autologous blood salvaged during surgery to restore their blood volume and homeostasis during the course of an operation when the blood is kept in a continuous circuit and connected at all times.
The global medical community has increasingly moved from allogeneic blood (blood collected from another person) towards autologous infusion...
Bloodless Options
Ways to avoid the adverse events associated with allogeneic transfusion are often grouped under the umbrella term bloodless surgery. There are several so-called bloodless options. These include minimally invasive surgical techniques; erythropoietin (a hormone that stimulates peripheral stem cells in the bone marrow to produce red blood cells); blood substitutes such as blood volume expanders and oxygen carriers (the latter as yet unlicensed in North America); autologous blood donation, including pre-operative donation (suitable only for scheduled surgery in which transfusion is anticipated) and intraoperative autologous donation or acute normovolemic hemodilution and blood salvage.
Intraoperative blood salvage has been used for many years, especially in cardiothoracic and vascular surgery, where blood usage has traditionally been high.
Blood Salvage Procedures
Several processes have been developed to assist in salvaging the patient's own whole blood in the perioperative setting. These can be categorized into three general types of salvage procedures:
- Cell processors and salvage devices that wash and save red blood cells, i.e.,
"cell washers" or RBC-savers - Direct transfusion
- Ultrafiltration of whole blood
Regardless of manufacturer, there are many types of cell processors. Cell processors are red cell washing devices that collect anticoagulated shed or recovered blood, wash and separate the red blood cells (RBCs) by centrifugation, and re-infuse the RBCs. RBC washing devices can help remove by-products in salvaged blood such as activated cytokines, anaphylatoxins, and other waste substances that may have been collected in the reservoir suctioned from the surgical field. However, they also remove viable platelets, clotting factors and other plasma proteins essential to whole blood and homeostasis. The various RBC-savers also yield RBC concentrates with different characteristics and quality.
Direct transfusion is a blood salvaging method associated with cardiopulmonary bypass (CPB) circuits or other extracorporeal circuits (ECC) that are used in surgery such as coronary artery bypass grafts (CABG), valve replacement, or surgical repair of the great vessels. Following bypass surgery the ECC circuit contains a significant volume of diluted whole blood that can be harvested in transfer bags and re-infused into patients. Residual CPB blood is fairly dilute ([Hb] = 6–9 g/dL; 60–90 g/L) and can also contain potentially harmful contaminants such as activated cytokines, anaphylatoxins, and other waste substances that have been linked to organ edema and organ dysfunction and need a diuretic to reverse.
Hemofiltration or ultrafiltration devices constitute the third major type of blood salvage appearing in operating rooms. In general, ultrafiltration devices filter the patient's anticoagulated whole blood. The filter process removes unwanted excess non-cellular plasma water, low molecular weight solutes, platelet inhibitors and some particulate matter through hemoconcentration, including activated cytokines, anaphylatoxins, and other waste substances making concentrated whole blood available for reinfusion.
Hemofilter devices return the patient's whole blood with all the blood elements and fractions including platelets, clotting factors, and plasma proteins with a substantial Hb level. These devices do not totally remove potentially harmful contaminants that can be washed away by most RBC-savers. However, the contaminants that are potentially reduced by using RBC-savers, as shown by data from in vitro laboratory tests, are transient and reversible in vivo with hemostatic profiles returning to baselines within hours. The key is that coagulation and homeostasis are immediately improved with the return of concentrated autologous whole blood.
Over the years numerous studies have been done to compare these methods of blood salvage in terms of safety, patient outcomes, and cost effectiveness, often with equivocal or contradictory results.1-4
Hemofilter devices return the patient's whole blood with all the blood elements and fractions including platelets, clotting factors, and plasma proteins with a substantial Hemoglobin level.
The Hemobag®
The Hemobag® is a new type of ultrafiltration reservoir designed to overcome the limitations of RBC-savers and direct retransfusion in cardiac, vascular, and other types of surgery through hemofiltration. The methodology of blood salvaging with the Hemobag® in the operating room is depicted in this video.
Being a new ultrafiltration method, the Hemobag® was not included in earlier papers and studies. Studies to date have shown the Hemobag® to quickly and safely recover substantial proteins, clotting factors, and red cell concentrates. 5-10
- Boldt J, Zickmann B, Fedderson B, Herold C, Dapper F, Hempelmann G. Six different hemofiltration devices for blood conservation in cardiac surgery. Ann Thorac Surg 1991 May;51(5):747-53.
- Sutton RG, Kratz JM, Spinale FG, Crawford FA Jr. Comparison of three blood-processing techniques during and after cardiopulmonary bypass. Ann Thorac Surg 1993 Oct;56(4):938-43.
- Eichert I, Isgro F, Kiessling AH, Saggau W. Cell saver, ultrafiltration and direct transfusion: comparative study of three blood processing techniques. Thorac Cardiovasc Surg 2001 Jun;49(3):149-52.
- Ferguson, J. How Ultrafiltration Works Discovery Health, 2011.
- Freischlag JA. Intraoperative blood salvage in vascular surgery - worth the effort? Crit Care 2004;8 Suppl 2:S53-6.
- Roeder B, Graham S, Searles B, Darling E. Evaluation of the Hemobag: a novel ultrafiltration system for circuit salvage. J Extra Corpor Technol 2004 Jun;36(2):162-5.
- Samolyk KA, Beckmann SR, Bissinger RC. A new practical technique to reduce allogeneic blood exposure and hospital costs while preserving clotting factors after cardiopulmonary bypass: the Hemobag. Perfusion 2005 Oct;20(6):343-9. [
 full text ]
full text ]  Reducing allogeneic blood exposure and preserving blood cell, protein and clotting factor concentration during cardiac surgery: Update on the Hemobag®(Society for the Advancement of Blood Management International Meeting, Phoenix, Sept. 2005)
Reducing allogeneic blood exposure and preserving blood cell, protein and clotting factor concentration during cardiac surgery: Update on the Hemobag®(Society for the Advancement of Blood Management International Meeting, Phoenix, Sept. 2005)- Moskowitz, DM, Klein JJ, Shander A, Perelman SI, McMurtry KA, Cousineau KM, Ergin MA.
 Use of the Hemobag® for modified ultrafiltration in a Jehovah's Witness patient undergoing cardiac surgery. JECT 2006;38:265–70.
Use of the Hemobag® for modified ultrafiltration in a Jehovah's Witness patient undergoing cardiac surgery. JECT 2006;38:265–70. - Beckmann SR, Carlile D, Bissinger RC, Burrell M, Winkler T, Shely WW. Improved coagulation and blood conservation in the golden hours after cardiopulmonary bypass. J Extra Corpor Technol 2007 Jun;39(2):103-8.
- Riley JB, Samolyk KA.
 On-line autotransfusion waste calculator. JECT 2008;40:68-73.
On-line autotransfusion waste calculator. JECT 2008;40:68-73.
External Links:
Note: Global Blood Resources LLC also created this article as a Wikipedia entry, where it appears in an altered form as Intraoperative Blood Salvage.